Bacteriophage therapy
Bacteriophage Therapy: A Promising Alternative to Antibiotics in the Fight Against Superbugs
As antibiotic resistance continues to rise at an alarming rate, the global medical community faces a growing crisis: once-treatable bacterial infections are becoming increasingly difficult — and sometimes impossible — to cure. In this urgent context, scientists and clinicians are revisiting a century-old idea with renewed interest: **bacteriophage therapy**. This innovative approach uses naturally occurring viruses called **bacteriophages (or phages)** to target and destroy pathogenic bacteria, offering a highly specific and potentially powerful alternative to traditional antibiotics.
What Are Bacteriophages?
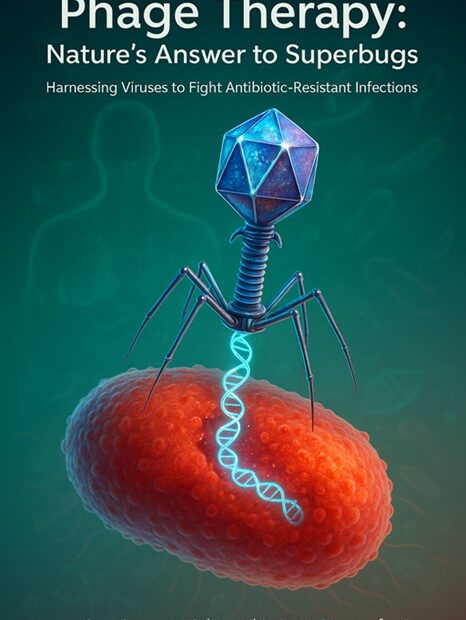
Bacteriophages, literally meaning “bacteria eaters,” are viruses that infect and replicate within bacteria. First discovered in the early 20th century by scientists like Félix d’Hérelle and Frederick Twort, phages are among the most abundant biological entities on Earth. They are found in diverse environments — from soil and seawater to the human gut — wherever bacteria exist.
Phages work by attaching to specific receptors on the surface of a bacterial cell, injecting their genetic material, and hijacking the host’s cellular machinery to produce new viral particles. This process ultimately leads to the lysis (bursting) of the bacterial cell, releasing new phages to continue the cycle and eliminate more bacteria.
How Does Phage Therapy Work?
Bacteriophage therapy involves isolating and administering phages that specifically target disease-causing bacteria. Unlike broad-spectrum antibiotics, which can wipe out both harmful and beneficial bacteria, phages are often **highly specific**, attacking only certain strains of bacteria. This precision allows phage therapy to eradicate pathogens while preserving the body’s healthy microbiota — a major advantage in preventing secondary infections and maintaining immune health.
The treatment process is often personalized. When a patient presents with a resistant bacterial infection, a sample of the pathogen is analyzed, and a matching phage (or cocktail of phages) is selected or engineered from phage libraries. Once identified, the phages are purified and administered — via injection, topical application, or oral ingestion — depending on the infection site.
A Solution to Antibiotic Resistance?
The rise of **multidrug-resistant bacteria** — such as MRSA (methicillin-resistant *Staphylococcus aureus*), *Pseudomonas aeruginosa*, and carbapenem-resistant *Enterobacteriaceae* — has created a pressing need for new antimicrobial strategies. Phage therapy offers a promising solution, particularly for patients with chronic or life-threatening infections who have exhausted all antibiotic options.
Notable success stories have emerged in recent years. For example, in 2016, a team at the University of California, San Diego used intravenous phage therapy to save a patient suffering from a systemic, antibiotic-resistant *Acinetobacter baumannii* infection. Similar compassionate-use cases have been reported worldwide, reigniting interest in phage therapy as a viable clinical tool.
Advantages of Phage Therapy
1. **High Specificity**: Phages target only specific bacteria, reducing collateral damage to the microbiome.
2. **Self-Replicating**: Phages multiply at the site of infection, potentially requiring lower initial doses.
3. **Adaptable**: Phage cocktails can be updated as bacteria evolve resistance, much like updating antivirus software.
4. **Natural and Abundant**: Phages are naturally occurring and can be isolated from diverse environments.
Despite its promise, phage therapy is not yet widely approved in most countries, including the United States and much of Western Europe. Several challenges remain:
– **Regulatory Hurdles**: Current drug approval frameworks are designed for standardized pharmaceuticals, not personalized, biologically variable treatments like phage cocktails.
– **Limited Clinical Trials**: While anecdotal evidence and case studies are encouraging, large-scale, randomized clinical trials are still needed to establish safety, efficacy, and dosing protocols.
– **Narrow Spectrum**: While specificity is an advantage, it also means that phages must be carefully matched to the infecting strain, requiring rapid diagnostics and access to diverse phage banks.
– **Potential for Resistance**: Bacteria can develop resistance to phages, though this can be mitigated using phage cocktails or combining phages with antibiotics.
Global Use and Future Outlook
Phage therapy has been used clinically for decades in countries like Georgia, Russia, and Poland, where institutions such as the Eliava Institute in Tbilisi have pioneered its development. In recent years, regulatory agencies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have begun to explore pathways for phage therapy approval, especially for compassionate use in life-threatening cases.
Research is also advancing in **phage engineering**, synthetic biology, and **phage-antibiotic synergy**, where the two treatments are combined to enhance bacterial killing and reduce resistance development.
Conclusion
Bacteriophage therapy represents a paradigm shift in the treatment of bacterial infections — one that embraces the complexity of microbial ecosystems rather than disrupting them. As antibiotic resistance threatens to return medicine to a pre-antibiotic era, phage therapy offers a beacon of hope. While more clinical evidence and regulatory frameworks are needed, the potential of these “smart viruses” to save lives in the age of superbugs is undeniable. With continued research, collaboration, and innovation, phage therapy may soon move from the fringes of medicine to the forefront of infectious disease treatment.
To get more info from the experts, reach out to: phagepakistan.com
Pakistan Society for Bacteriophages (PSB) aims to leverage the collective expertise and resources at the national and international levels.